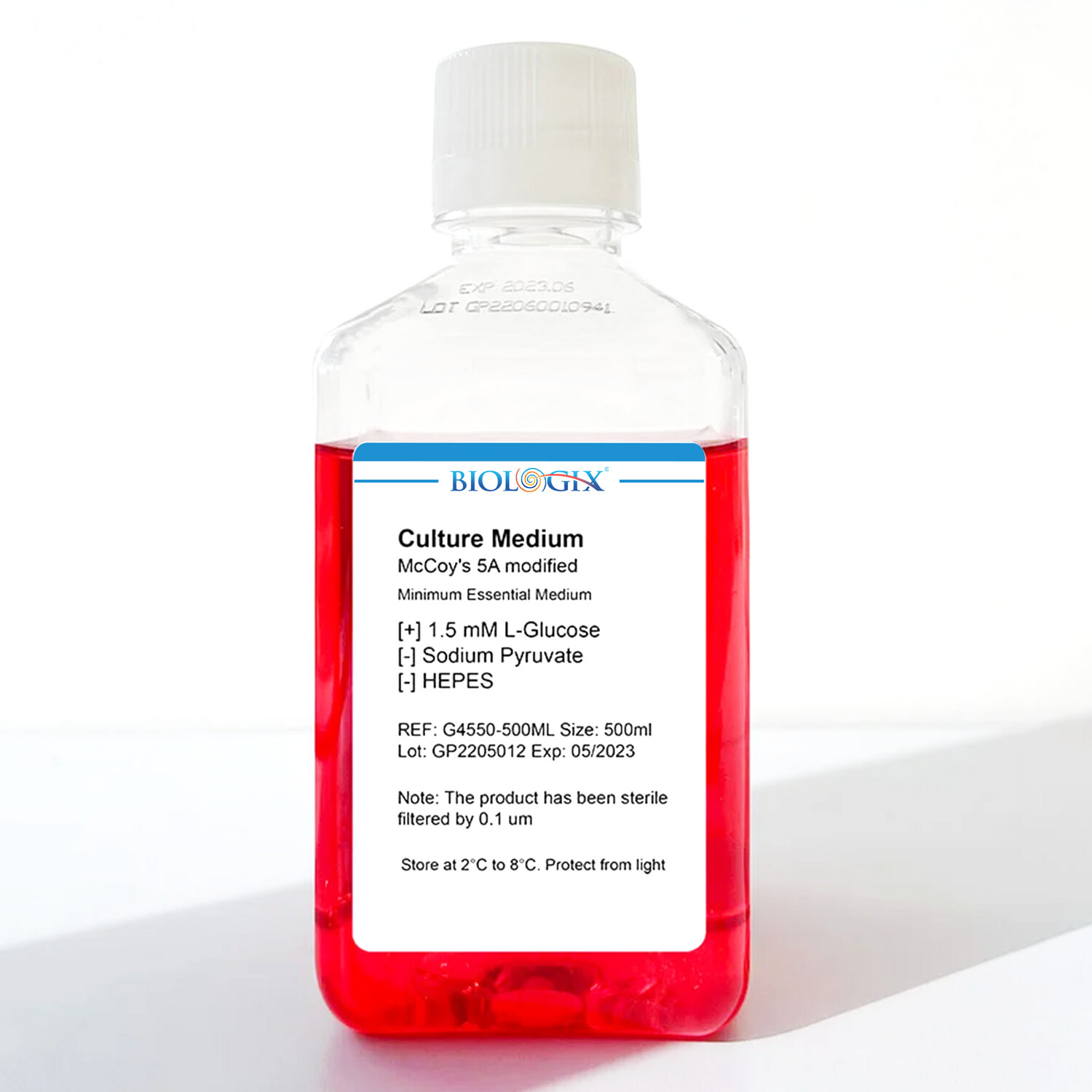
McCoy's 5A Modified Culture Medium, 500 ML
Download Product Document:
Features:
This product is filtered and sterilized by 0.1μm filter membrane, containing 25 mM glucose, glutamine and sodium BT, without HEPES. Dr. Thomas McCoy originally formulated McCoy’s 5A medium as a modification of Basal Medium 5A. Different from other media, McCoy’s 5A contains the reducing glutathione, bacto-peptone, a high level of glucose, and also includes Hanks’s salts to enable use outside a CO2 incubator. McCoy’s 5A medium modified generally supports the propagation of many types of primary mammalian cells derived from normal bone marrow, skin, spleen, kidney, lung rat embryos and other tissues. It can also be used for growth of established cell lines and explants from biopsy tissues. McCoy’s 5A medium modified contains no proteins, lipids, or growth factors. Therefore, McCoy’s 5A medium modified requires supplementation, commonly with 10% Fetal Bovine Serum (FBS). McCoy’s 5A medium modified uses a sodium bicarbonate buffer system (2.2 g/L) and therefore requires a 5–10% CO2 environment to maintain physiological pH. All raw material components used for Hygia’s cell culture media products are screened through using strict quality control testing. Hygia’s cell culture media are manufactured at a cGMP-compliant facility using water for injection, filtered through final 0.1 μm sterile filtration and aseptic filled in a Hundred-stage FFU. The filter integrity tests are performed before and after filtration. Each lot of cell culture media is subjected to sterility test, pH, osmolality, and cell culture test.
| Overview | |
| Composition | 16.67 mM glucose, 1.5mM L glutamine, phenol red |
| Does NOT contain | Without sodium pyruvate and HEPES |
| Storage condition | 2℃ to 8℃, protect from light |
| Shipping condition | ambient |
| Shelf life | 12 months from date of manufacture |